| [1] |
YEAGER K. Improvised explosives characteristics. In: Beveridge A, editor. Forensic investigation of explosions[M]. 2nd edn. Boca Raton: CRC Press Taylor&Francis Group, 2012: 493-538.
|
| [2] |
ARMITT D, ZIMMERMAN P, ELLIS-STEINBORNER S. Gas chromatography/mass spectrometry analysis of triacetone triperoxide (TATP) degradation products[J]. Rapid Commun Mass Spectrom, 2008, 22: 950-958.
|
| [3] |
DUNN L, OBAIDLY H S A A, KHALIL S E. Development and validation of fast liquid chromatography high-resolution mass spectrometric (LC-APCI-QToFMS) methods for the analysis of hexamethylene triperoxide diamine (HMTD) and triacetone triperoxide (TATP)[J]. Forensic Chem, 2018, 10: 5-14.
|
| [4] |
JIANG D, PENG L, WEN M, ZHOU Q, CHEN C, WANG X, CHEN W, LI H. Dopant-assisted positive photoionization ion mobility spectrometry coupled with time-resolved thermal desorption for on-site detection of TATP and HMTD in complex matrices[J]. Anal Chem, 2016, 88(8): 4391-4399.
|
| [5] |
BOTTI S, CANTARINI L, PALUCCI A. Surface-enhanced Raman spectroscopy for trace-level detection of explosives[J]. J Raman Spectrosc, 2010, 41(8): 866-869.
|
| [6] |
BERGLUND M, WIESER M E. Isotopic compositions of the elements 2009[J]. Pure Appl Chem, 2011, 83(2): 397-410.
|
| [7] |
韩娟,刘汉彬,金贵善,张建锋,李军杰,张佳,石晓. 方解石-白云石混合物碳、氧同位素组成的Gasbench-IRMS选择性酸提取法研究[J]. 质谱学报,2022,43(2):210-219.
HAN Juan, LIU Hanbin, JIN Guishan, ZHANG Jianfeng, LI Junjie, ZHANG Jia, SHI Xiao. Carbon and oxygen lsotope analysis of calcite and dolomite mixtures using selective acid extraction by Gasbench-IRMS[J]. Journal of Chinese Mass Spectrometry Society, 2022, 43(2): 210-219(in Chinese).
|
| [8] |
QIE M J, LI T W, LIU C C, ZHAO Y. Direct analysis in real time high-resolution mass spectrometry for authenticity assessment of lamb[J]. Food Chemistry, 2022, 390: 133 143.
|
| [9] |
马玉华,唐方东,刘佳煜,忻智炜,曾友石,杜林,刘卫. 稳定同位素比技术用于橄榄油的掺假鉴定[J]. 质谱学报,2021,42(2):189-196.
MA Yuhua, TANG Fangdong, LIU Jiayu, XIN Zhiwei, ZENG Youshi, DU Lin, LIU Wei. Olive oil adulteration identification using stable lsotope ratio technique[J]. Journal of Chinese Mass Spectrometry Society, 2021, 42(2): 189-196(in Chinese).
|
| [10] |
BERENS M J, HOFSTETTER T B, BOLOTIN J, ARNOLD W A. Assessment of 2,4-dinitroanisole transformation using compound-specific isotope analysis after in situ chemical reduction of iron oxides[J]. Environ Sci Technol, 2020, 54(9): 5520-5531.
|
| [11] |
胡灿,梅宏成,郭洪玲,孙振文,刘占芳,朱军. 常见炸药的稳定同位素比值分析方法研究进展[J]. 色谱,2021,39(4):376-383.
HU Can, MEI Hongcheng, GUO Hongling, SUN Zhenwen, LIU Zhanfang, ZHU Jun. Recent advances in stable isotope ratio analysis of common explosives[J]. Chinese Journal of Chromatography, 2021, 39(4): 376-383(in Chinese).
|
| [12] |
MICHAEL B, JAKOV B, THOMAS B H. Compound-specific nitrogen and carbon isotope analysis of nitroaromatic compounds in aqueous samples using solidphase microextraction coupled to GC/IRMS[J]. Anal Chem, 2007, 79: 2386-2393.
|
| [13] |
HU C, MEI H C, GUO H L, YU Z Y, ZHU J. Purification of ammonium nitrate via recrystallization for isotopic profiling using isotope ratio mass spectrometry[J]. Forensic Sci Int, 2021, 328: 111 009.
|
| [14] |
HUANG Y, ZHU J, HU C, MEI H C, YU Z Y, QIN H. Desulfurization of black powder for isotopic profiling using isotope ratio mass spec-trometry[J]. Forensic Sci Int, 2022, 337: 111 379.
|
| [15] |
BENSON S J, LENNARD C J, MAYNARD P, HILL D M, ANDREW A S, ROUX C. Forensic analysis of explosives using isotope ratio mass spectrometry (IRMS)preliminary study on TATP and PETN[J]. Sci Justice, 2009, 49: 81-86.
|
| [16] |
BEZEMER K D B, KOEBERG M, ANTOINE E D M, HEIJDEN V D, DRIEL C A V, BLAGA C, BRUINSMA J, ASTEN A C V. The potential of isotope ratio mass spectrometry (IRMS) and gas chromatography-IRMS analysis of triacetone triperoxide in forensic explosives investigations[J]. J Forensic Sci, 2016, 61(5): 1198-1207.
|
| [17] |
FAINA D, RONNIE K, JOSEPH A, YEHUDA Z, ROLAND B, HAREL I, AARON A, EHUD K. Decomposition of triacetone triperoxide is an entropic explosion[J]. J Am Chem Soc, 2005, 127: 1146-1159.
|
| [18] |
CARTER J F, BARWICK V J. Good practice guide for isotope ratio mass spectrometry[M]. UK: the FIRMS Network, 2011: 1-35.
|
| [19] |
OXLEY J C, SMITH J L, STEINKAMP L, ZHANG G. Factors influencing triacetone triperoxide (TATP) and diacetone diperoxide (DADP) formation: part 2[J]. Propellants, Explos, Pyrotech, 2013, 35: 1-11.
|
| [20] |
MATYAS R, PACHMAN J. Primary explosives[M]. Berlin: SpringerVerlag, 2013: 255-288.
|
| [21] |
BENSON S, LENNARD C, MAYNARD P, ROUX C. Forensic applications of isotope ratio mass spectrometry-a review[J]. Forensic Sci Int, 2006, 157: 1-22.
|



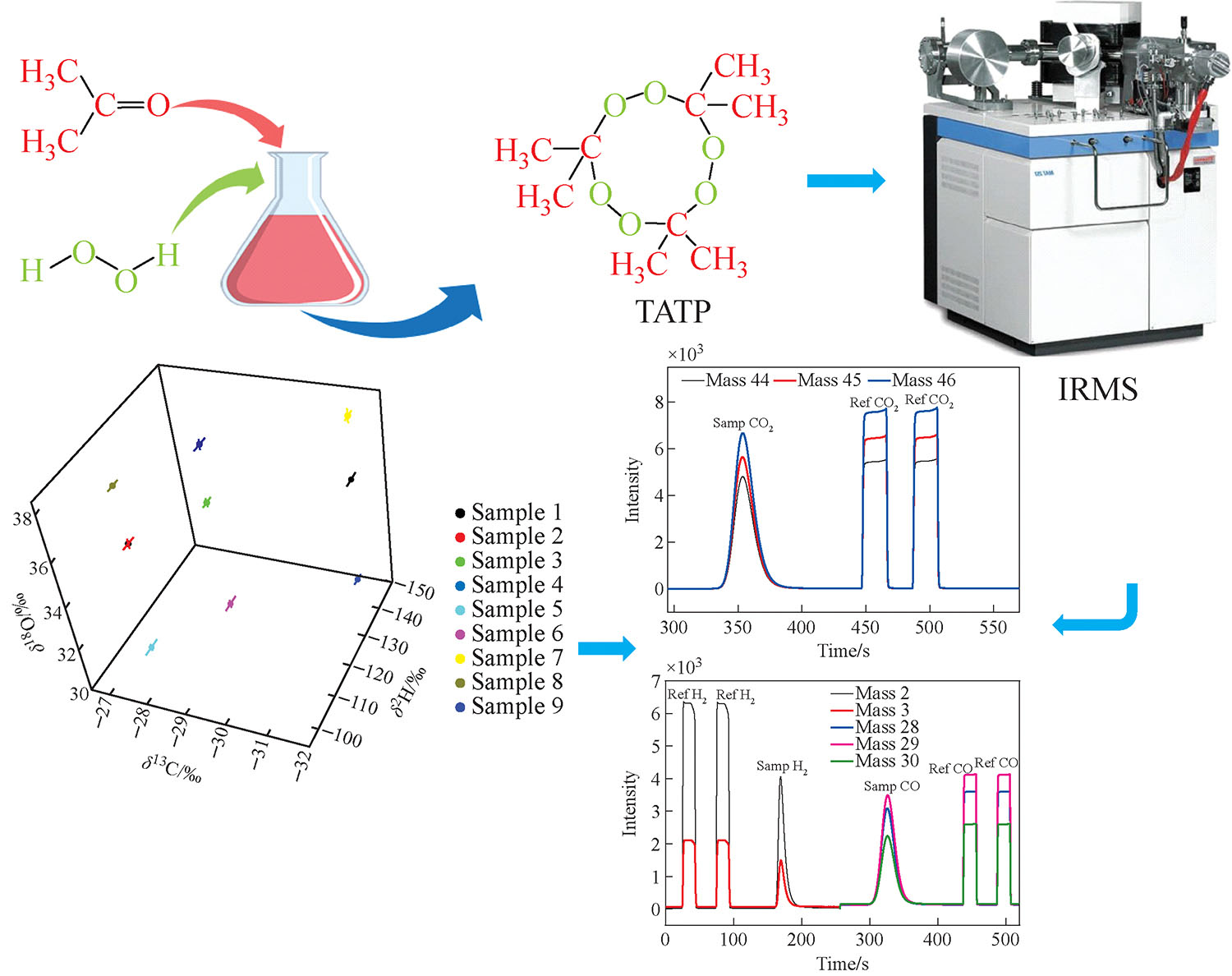
 下载:
下载:
